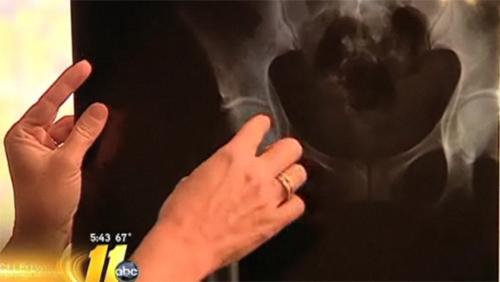
 Even after a heavy year for medical product recalls, medical device companies have vamped up their criticism of government regulation, and are actively seeking to undermine efforts to make safer products. Last week, for example, the New York Times mentioned DePuy faulty hip implants in an article about companies who say the safety regulations will send business overseas. But a study released this week has shown that the Food and Drug Administration used an abbreviated approval process for more than 70 percent of the products that were recalled over the past five years. For good reason, this study is likely to dampen any hopes for decreased FDA scrutiny.
Even after a heavy year for medical product recalls, medical device companies have vamped up their criticism of government regulation, and are actively seeking to undermine efforts to make safer products. Last week, for example, the New York Times mentioned DePuy faulty hip implants in an article about companies who say the safety regulations will send business overseas. But a study released this week has shown that the Food and Drug Administration used an abbreviated approval process for more than 70 percent of the products that were recalled over the past five years. For good reason, this study is likely to dampen any hopes for decreased FDA scrutiny.
The approval process in question allows a new product to undergo an abbreviated safety review if it shown to be similar to another product already on the market. DePuy hip implants, for example, were similar to the Ultima model, which had been approved years earlier, and therefore was able to sail through the FDA approval process. Apparently this wasn’t such a good idea, as the Archives of Internal Medicine study found that 70 percent of the 113 recalled devices over the past five years were part of the shorter review. And if regulation doesn’t get stronger, we can expect even more recalls, said Diana Zuckerman, one of the study’s co-authors: “The law has gotten looser and looser over time.”
For the most part, the FDA has remained defensive. “Even one recall is too many,” spokeswoman Karen Riley told the Washington Post. “But considering that more than 19,000 devices were cleared via…the process between 2005 and 2009, it’s important to keep the 80 recalls in perspective. They represent a small number of the devices cleared via the program and don’t reflect the thousands of people who have benefited from these devices.” But this argument fell flat with cardiologist and co-author Dr. Steven E. Nissen, who said simply “this is an area where the FDA has failed the public.”
DePuy lawsuits likely won’t heat up for months or even years. But in the mean time, journalists and scientists will continue releasing information about DePuy hip implants that will shed light onto the question of whether the Johnson & Johnson subsidiary rushed a dangerous hip implant product onto the market. We’ll keep you posted.



 AI-search
AI-search  Email
Email